In addition to collaborating with traditional healthcare providers, Gelesis will leverage Ro’s nationwide telehealth services to make Plenity available to patients who don’t have an established healthcare provider or prefer a remote interaction
Ro provides a personalized end to end telehealth experience from medical diagnosis to the home delivery of prescription and OTC products
Plenity will be the only weight management treatment available through Ro’s digital clinics as Ro expands to treat new conditions on its platform
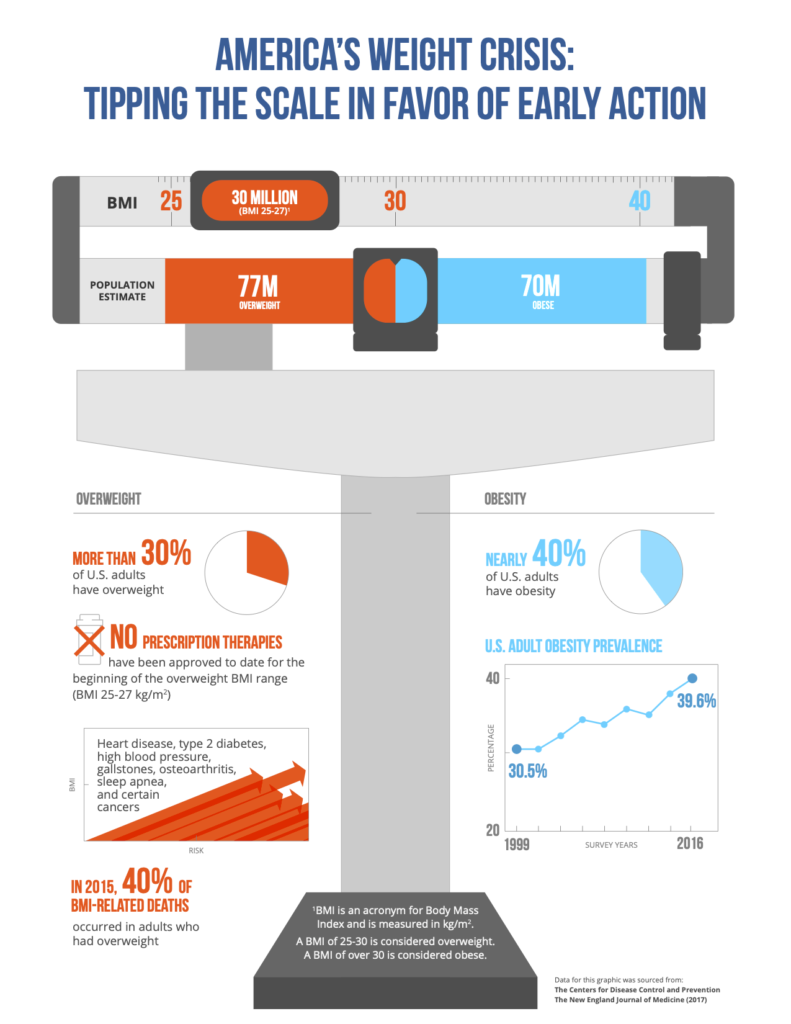
BOSTON, Dec. 17, 2019 — Gelesis, a biotechnology company developing a novel hydrogel platform technology to treat obesity and other chronic diseases related to the gastrointestinal (GI) tract, and Ro, a leading U.S. telehealth provider, announced today they will partner to offer high-quality remote care dedicated to weight management and prescription fulfillment of Plenity. Ro’s telehealth services will complement traditional live interactions to ensure people seeking healthcare provider guidance for weight loss will have easy access to multiple options. Through the Gelesis-Ro partnership, Plenity will be the first FDA-cleared weight management aid and first primary care product to launch with both traditional healthcare provider and telehealth services, making Plenity available to patients nationwide.
Plenity, an orally administered, non-systemic and non-stimulant aid to weight management, was recently cleared for use by the FDA in adults with a Body Mass Index (BMI) of 25–40 kg/m , when used in conjunction with diet and exercise. It is the only prescription weight management product indicated for use by overweight adults with a BMI as low as 25 kg/m , making it available for the largest number of adults affected by overweight and obesity of any prescription weight-management aid.
Ro currently powers digital health clinics that provide a personalized end to end telehealth experience from medical diagnosis to the delivery of prescription and over-the-counter products. Plenity will be the only weight management treatment available through Ro’s digital clinics as the company expands to treat new conditions on its platform. Ro’s portfolio currently includes products for men’s and women’s sexual health, smoking cessation and insomnia.
Obesity is a chronic progressive disease resulting from multiple environmental and genetic factors. Because of the disease complexity, there are numerous therapeutic and social challenges facing individuals seeking assistance to achieve a healthier weight. There are also limited safe and effective prescription options to treat adults who are overweight but do not meet the clinical threshold for obesity.
“Our current healthcare system is ill-equipped to assist the millions of patients seeking to manage their weight, especially starting at an overweight BMI of 25,” said Caroline Apovian, M.D., FACP, FACN, FTOS, professor of medicine and pediatrics in the Section of Endocrinology, Diabetes, Nutrition and Weight Management at Boston University School of Medicine and outgoing president of the Obesity Society. “It is important to support effective interactions between patients and their physicians regarding weight management while providing new modalities of access to care for patients who do not have an established healthcare provider or for those who prefer a different type of interaction.”
Through Ro’s telehealth platform, adults will have access to physicians trained in weight management support. Those interested in learning if Plenity is a safe and appropriate treatment for them can seek care through an online doctor’s visit dedicated to weight management. If it is determined that an individual should be seen in person for follow-up care or is better suited for another course of treatment, the provider will recommend that they visit their primary care provider or a specialist. Adults who receive a prescription for Plenity through Ro’s platform will have access to unlimited follow up communication with their physician at no additional cost and will be provided with educational resources and lifestyle recommendations. Ro can also keep a patient’s in-person provider updated on their treatment plan at the patient’s request to ensure continuity of care.
“Through the millions of patient-physician interactions facilitated on our platform, physicians have seen the dramatic and rippling impact of weight-related health issues firsthand,” said Zachariah Reitano, co- founder and chief executive officer of Ro. “Prior to this point, safe and effective treatment options for weight management were limited and patients have faced significant barriers to accessing high-quality care—whether that’s due to stigmatization, cost or geography. Through this unique partnership, Ro will provide high-quality, innovative treatment for weight management, deliver Plenity straight to a patient’s door, and maintain unrivaled care on one seamless platform. Together, we can have a profound impact on how patients and physicians tackle this serious and pervasive health challenge.”
“Our partnership with Ro reflects our shared vision of improving access for patients in all corners of the country, including those who may not feel comfortable talking to their primary care physician about weight management,” said David Pass, Pharm.D., Gelesis head of commercial and chief operating officer. “The objective remains simple: give people more choices, more support and more power to manage their weight. We believe Ro is uniquely suited to provide telehealth services that enable more individuals to access the high-quality care they need, providing an additional path to treatment and support for the complex, chronic health conditions of overweight and obesity.”
Gelesis retains worldwide sales and marketing rights for Plenity and, since FDA clearance, has secured close to $100 million in new capital to support the U.S. commercial launch. Gelesis initiated an early experience program as part of its targeted U.S. launch of Plenity earlier this year and expects that Plenity will be available in limited supply through healthcare providers and Ro’s digital health clinics in the second half of 2020. In parallel, the Company has been expanding its commercial manufacturing capabilities and will continue to add capacity to meet demand.
About PLENITY™
PLENITY is an oral, non-systemic, superabsorbent hydrogel which has received FDA clearance as an aid in weight management in overweight and obese adults with a BMI of 25–40 kg/m , when used in conjunction with diet and exercise. It is made by cross-linking two naturally derived building blocks, modified cellulose and citric acid, that create a three-dimensional matrix. PLENITY particles rapidly absorb water in the stomach and homogenously mix with ingested foods. Rather than forming one large mass, it creates thousands of small individual gel pieces with the elasticity (firmness) of solid plant-based foods (e.g., vegetables) without caloric value. The PLENITY hydrogel increases the volume and elasticity of the stomach and small intestine contents and induces a feeling of fullness and satiety. Once it arrives in the large intestine, the hydrogel is partially broken down by enzymes and loses its three-dimensional structure along with most of its absorption capacity. The released water is reabsorbed in the large intestine, and the remaining cellulosic material is eliminated through the body’s natural digestive processes. PLENITY is considered a medical device because it achieves its primary intended purpose through mechanical modes of action consistent with mechanobiology constructs. For more information, visit myplenity.com.
Important Safety Information
- PLENITY is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin or titanium oxide.
- PLENITY may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully.
- Avoid use in patients with the following conditions: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); or complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility.
- Use with caution in patients with active GI conditions such as gastro-esophageal reflux disease (GERD), ulcers or heartburn.
- Overall, the most common treatment related adverse events (TRAEs) were GI-related with 38% of adults in the PLENITY group and 28% of adults in the placebo group.
- The overall incidence of adverse events (AEs) in the PLENITY group was no different than the placebo group.
Rx Only. For the safe and proper use of PLENITY, refer to the Instructions for Use.
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of certain chronic diseases. In April 2019, Gelesis received FDA clearance for its lead product candidate, Plenity™, as an aid for weight management in overweight and obese adults with a Body Mass Index (BMI) of 25-40 kg/m , when used in conjunction with diet and exercise. Gelesis anticipates Plenity will be available by prescription in the U.S. in the second half of 2020. Additionally, Gelesis is developing its second investigational candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel mechanotherapeutics based on the Gelesis platform technology are also being advanced in other GI inflammatory conditions, such as non-alcoholic steatohepatitis (NASH) and Chronic Idiopathic Constipation (CIC).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials science, chronic disease research, and commercialization. Gelesis was co-founded by PureTech Health (LSE: PRTC), a clinical-stage biotechnology company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases. For more information, visit gelesis.com or connect with us on Twitter @GelesisInc.
About Ro
Ro is a patient-driven healthcare company that puts you in control of your health. We’re patients, just like you, building technology to make healthcare accessible, affordable, and maybe even enjoyable. Today, we enable you to connect with a doctor and get treatment when and where you need it. Tomorrow, we aspire to be your first call for all your healthcare needs. Ro powers three digital health clinics — Roman, Rory and Zero — providing a personalized end to end telehealth experience from diagnosis to delivery. Ro’s platform has facilitated over 3 million doctor-patient interactions through its secure healthcare portal and is live in all 50 states.
Contact:
Investors
Allison Mead Talbot
+1 617 651 3156 amt@puretechhealth.com
U.S. media
Tom Donovan
+1 857 559 3397 tom@tenbridgecommunications.com
Ro
Meghan Pianta +1 203 556 5970 meghan@ro.co
Vitruvian Partners leads a $63.4 million equity round, complemented by $21.2 million in new, non- dilutive grant funding and loans to further support commercialization efforts
BOSTON, Dec. 9, 2019 — Gelesis, a biotechnology company developing a novel hydrogel platform technology to treat obesity and other chronic diseases related to the gastrointestinal (GI) tract, today announced it secured $84.6 million in new capital. In total, Gelesis has obtained nearly $100 million this
TM year to support the U.S. launch of Plenity™.
This latest round of equity funding totaling $63.4 million was led by private equity firm Vitruvian Partners and included other investors. The proceeds from the financing will be used primarily to support the U.S. launch of Plenity in the second half of 2020.
“We are delighted to begin this partnership with Vitruvian, whose mission of driving rapid growth and change across industries is very much aligned with our approach to launching this first-of-its-kind product that could potentially make a difference in the lives of people struggling with excess weight,” said Yishai Zohar, founder and chief executive officer of Gelesis. “With this new capital, we are well-positioned to enhance our strategic launch initiatives and leverage our early commercial experience as we prepare for large scale commercial availability of Plenity in the U.S.”
Based upon the Gelesis’ proprietary manufacturing facility location, the company was also awarded a grant of $12.9 (€11.7) million from the European Regional Development Fund (ERDF), regulated by the Puglia Region of Italy. This builds on the $10.6 (€9.4) million grant announced in April 2019 and brings the total non-dilutive funds secured this year to $23.5 million. The company also further enhanced its financial flexibility by entering into a long-term, low interest $8.3 million loan agreement.
About PLENITY™
PLENITY is an oral, non-systemic, superabsorbent hydrogel which has received FDA clearance as an aid in weight management in overweight and obese adults with a BMI of 25–40 kg/m , when used in conjunction with diet and exercise. It is made by cross-linking two naturally derived building blocks—modified cellulose and citric acid—that create a three-dimensional matrix. PLENITY particles rapidly absorb water in the stomach and homogenously mix with ingested foods. Rather than forming one large mass, it creates thousands of small individual gel pieces with the elasticity (firmness) of solid plant-based foods (e.g., vegetables) without caloric value. The PLENITY hydrogel increases the volume and elasticity of the stomach and small intestine contents and induces a feeling of fullness and satiety. Once it arrives in the large intestine, the hydrogel is partially broken down by enzymes and loses its three-dimensional structure along with most of its absorption capacity. The released water is reabsorbed in the large intestine, and the remaining cellulosic material is eliminated through the body’s natural digestive processes. PLENITY is considered a medical device because it achieves its primary intended purpose through mechanical modes of action consistent with mechanobiology constructs. For more information, visit myplenity.com.
Important Safety Information
- PLENITY is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin or titanium oxide.
- PLENITY may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully.
- Avoid use in patients with the following conditions: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); or complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility.
- Use with caution in patients with active GI conditions such as gastro-esophageal reflux disease (GERD), ulcers or heartburn.
- Overall, the most common treatment related adverse events (TRAEs) were GI-related with 38% of adults in the PLENITY group and 28% of adults in the placebo group.
- The overall incidence of adverse events (AEs) in the PLENITY group was no different from the placebo group.
Rx Only. For the safe and proper use of PLENITY, refer to the Instructions for Use.
About Vitruvian Partners
Vitruvian is an international private equity firm headquartered in London with offices across London, Stockholm, Munich, Luxembourg, San Francisco and Shanghai. Vitruvian focuses on dynamic situations characterized by rapid growth and change across industries spanning information technology, financial services, life sciences & healthcare, media, and business and consumer services. Vitruvian is currently investing from its third fund, the €2.4 billion Vitruvian Investment Partnership III, which is among the largest pools of capital in Europe supporting innovative and higher growth companies. Vitruvian Funds have backed over 45 companies and have assets under management of approximately $5.5 billion. Notable investments to date include global market leaders in their field such as Just Eat, FarFetch, Darktrace, Trustpilot, Snow Software, TransferWise, Skyscanner and others. The Firm’s previous investments in life science innovators include companies such as doctari, CRF Health, ADA Health, Dental Monitoring. More information can be found at: www.vitruvianpartners.com
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of certain chronic diseases. In April 2019, Gelesis received FDA clearance for its lead product candidate, Plenity™, as an aid for weight management in overweight and obese adults with a Body Mass Index (BMI) of 25-40 kg/m , when used in conjunction with diet and exercise. Gelesis anticipates Plenity will be available by prescription in the U.S. in the second half of 2020. Additionally, Gelesis is developing its second investigational candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. This novel Gelesis hydrogel technology is also being advanced in other GI conditions, such as non-alcoholic steatohepatitis (NASH) and Chronic Idiopathic Constipation (CIC).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials science, chronic disease research, and commercialization. Gelesis was co-founded by PureTech Health (LSE: PRTC), a clinical-stage biotechnology company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases. For more information, connect with us on Twitter @GelesisInc.
Contact
Investors
Allison Mead Talbot
+1 617 651 3156 amt@puretechhealth.com
U.S. media
Tom Donovan
+1 857 559 3397 tom@tenbridgecommunications.com
Plenity-treated adults achieving a BMI of <27 lost an average of 13.5% of their weight with the rate of weight loss tapering as participants approached a healthy BMI goal
Twice as many adults reached a BMI of 27 or less when treated with Plenity compared to placebo
No increased safety risk observed in lower BMI adults (<35), with the overall incidence of treatment-related adverse events no different from placebo
BOSTON, Nov. 5, 2019 — Gelesis, a biotechnology company developing a novel hydrogel platform technology to treat obesity and other chronic diseases related to the gastrointestinal (GI) tract, today announced results of a new post-hoc analysis from the Gelesis Loss of Weight (GLOW) clinical trial for participants achieving a Body Mass Index (BMI) of 27 kg/m2 or less. These data showed that twice as many adults (11%) lost enough weight to achieve a BMI of 27 or less when treated with PlenityTM (Gelesis100) than when treated with placebo (5%). Plenity is an oral, non-systemic, superabsorbent hydrogel that rapidly absorbs water in the stomach and mixes homogeneously with ingested foods to increase the volume and elasticity of the stomach and small intestine contents. Consistent with the larger GLOW cohort, the overall incidence of adverse events (AEs) in lower-BMI adults treated with Plenity was no different from placebo treatment. The results were shared in an oral session at ObesityWeek 2019, the annual combined congress of the American Society for Metabolic and Bariatric Surgery and The Obesity Society.
Less than half of the approximately 150 million adults in the U.S. struggling with overweight and obesity (BMI 25 kg/m2 to 40 kg/m2) meet the clinical threshold for obesity (BMI > 30 kg/m2). Yet the health burden of excess weight begins before the onset of obesity, with approximately 40% of BMI-related deaths in 2015 occurring in overweight adults with a BMI <30 kg/m2. Studies show even modest weight gain in early adulthood is strongly associated with critical outcomes such as cancer risk and mortality.
“In order to break the cycle of adult obesity and have a meaningful impact on both individual and population health, we should shift the treatment paradigm to prevent obesity by treating patients when they are overweight and before they meet the clinical definition of obesity,” said Ken Fujioka, M.D., a weight loss expert, endocrinology researcher at Scripps Clinic and scientific advisor to Gelesis. “This subgroup analysis provides clear and compelling insight into the safety and efficacy of Plenity treatment in overweight patients with a lower-BMI, and – in conjunction with the exciting results from the overall study – provide a strong rationale for Plenity as an early therapeutic intervention for adults with excess weight.”
During an oral presentation at ObesityWeek 2019, study investigators delivered data from a new subgroup analysis of the Glow study assessing the safety and efficacy of Plenity in study participants reaching a BMI of <27 kg/m2. The mean BMI at baseline for this Plenity-treated subgroup was 29.9 +/- 1.56 SD. Within this subgroup, adults treated with Plenity, on average, lost 13.5% of their total body weight in approximately 100 days with the rate of weight loss tapering as participants approached a healthy BMI. After achieving a BMI of <27 kg/m2, participants continued Plenity treatment for an average of 60 days. The overall safety and tolerability profile of Plenity within this group was no different from placebo.
Gelesis Loss of Weight (GLOW) clinical study
The Gelesis Loss Of Weight (GLOW) Study was a randomized, double-blind, placebo-controlled, parallel-group study enrolling 436 adults with a body mass index (BMI) ≥ 27 and ≤ 40 kg/m2, including those with prediabetes or type 2 diabetes. The 6-month study compared a 2.25 g dose of Plenity, administered twice daily, to placebo and was conducted at 33 sites across the United States and several European countries. Both the active and placebo arms also included a hypocaloric diet and daily physical activity.
The study had two predefined co-primary endpoints: at least 35% of patients taking Plenity achieving ≥ 5% weight loss (categorical endpoint) and placebo-adjusted weight loss with a super-superiority margin of 3%. In addition, a prespecified analysis of simple superiority was also performed. The study met and exceeded the predefined categorical endpoint, with 59% of adults in the treatment group achieving weight loss of 5% or greater. As previously announced, the study did not meet the 3% super-superiority endpoint but demonstrated superiority of the Plenity treatment over the placebo group (–6.4% vs. –4.4%, P=0.0007). Plenity-treated individuals had twice the odds of achieving at least 5% weight loss vs. placebo (adjusted odds ratio [OR]: 2.0, P=0.0008).
In addition, 26% of the adults who completed the treatment with Plenity were “super-responders,” defined as achieving at least 10% weight loss. These super-responders achieved an average of about 14% weight loss or approximately 30 pounds.
The overall incidence of adverse events (AEs) in the Plenity treatment group was no different from placebo. The most common treatment-related adverse events (TRAEs) were gastrointestinal disorders (158 TRAEs in 84 [38%] subjects in the Plenity arm, compared to 105 events in 58 [28%] subjects receiving placebo), infections and infestations (2 events in 2 [1%] subjects with Plenity and 1 events in 1 [1%] subjects with placebo), and musculoskeletal and connective tissue disorders (3 events in 2 [1%] subjects with Plenity and 0 in 0 [0%] subjects with placebo). There were no serious adverse events (SAE) in the Plenity treatment group, whereas there was one (1) SAE in the placebo treatment group.
About Plenity™ (Gelesis100)
Plenity is an oral, non-systemic, superabsorbent hydrogel which has received FDA clearance as an aid in weight management in overweight and obese adults with a BMI of 25–40 kg/m2, when used in conjunction with diet and exercise. It is the only prescription therapeutic cleared by the FDA for use in overweight adults with a BMI below 30 kg/m2, with or without comorbidities such as hypertension, type 2 diabetes, and dyslipidemia. Plenity is made by cross-linking two naturally derived building blocks, modified cellulose and citric acid, that create a three-dimensional matrix. Plenity particles rapidly absorb water in the stomach and homogenously mix with ingested foods. Rather than forming one large mass, it creates thousands of small individual gel pieces with the elasticity (firmness) of solid plant-based foods (e.g., vegetables) without caloric value. The Plenity hydrogel increases the volume and elasticity of the stomach and small intestine contents and induces a feeling of fullness and satiety. Once it arrives in the large intestine, the hydrogel is partially broken down by enzymes and loses its three-dimensional structure along with most of its absorption capacity. The released water is reabsorbed in the large intestine, and the remaining cellulosic material is eliminated through the body’s natural digestive processes. Plenity is considered a medical device because it achieves its primary intended purpose through mechanical modes of action consistent with mechanobiology constructs. For more information, visit myplenity.com.
Important Safety Information
- Plenity is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin or titanium oxide.
- Plenity may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully.
- Avoid use in patients with the following conditions: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); or complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility.
- Use with caution in patients with active GI conditions such as gastro-esophageal reflux disease (GERD), ulcers or heartburn.
- Overall, the most common treatment-related adverse events (TRAEs) were GI-related, with 38% of adults in the Plenity group and 28% of adults in the placebo group.
- The overall incidence of adverse events (AEs) in the Plenity group was no different from the placebo group.
Rx Only. For the safe and proper use of Plenity, refer to the Instructions for Use.
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of certain chronic diseases. In April 2019, Gelesis received FDA clearance for its lead product candidate, Plenity™, as an aid for weight management in overweight and obese adults with a Body Mass Index (BMI) of 25-40 kg/m2, when used in conjunction with diet and exercise. Gelesis anticipates Plenity will be available by prescription in the U.S. in the second half of 2020. Additionally, Gelesis is developing its second investigational candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel mechanotherapeutics based on the Gelesis platform technology are also being advanced in other GI inflammatory conditions, such as non-alcoholic steatohepatitis (NASH) and Chronic Idiopathic Constipation (CIC).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials science, chronic disease research, and commercialization. Gelesis was co-founded by PureTech Health (LSE: PRTC), a clinical-stage biotechnology company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases. For more information, visit gelesis.com or connect with us on Twitter @GelesisInc.
Contact:
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
Gelesis ObesityWeek2019 – Download PDF
Relevance of intervention with Gelesis100 in overweight and mild obesity: a subgroup analysis of the pivotal GLOW study
Assessment of the safety and tolerability of Gelesis100 in subjects who reached a body mass index below 27 kg/m2in the pivotal GLOW study
Comprehensive analysis of safety and tolerability of Gelesis100 in overweight and obesity in the pivotal GLOW study
BOSTON, Oct. 29, 2019 — Gelesis, a biotechnology company developing a novel hydrogel platform technology to treat obesity and other chronic diseases related to the gastrointestinal (GI) tract, today announced that the Company will deliver two oral presentations and one poster at ObesityWeek 2019, the annual combined congress of the American Society for Metabolic and Bariatric Surgery and The Obesity Society in Las Vegas, Nevada, from November 3-7, 2019. The presentations will detail data regarding the safety and efficacy of PlenityTM (Gelesis100), including a new subgroup analysis of patients achieving a Body Mass Index (BMI) of at least 27 kg/m2 in the company’s pivotal Gelesis Loss of Weight (GLOW) clinical trial. Plenity is an oral, non-systemic therapeutic cleared by the FDA as an aid for weight management in adults with a Body Mass Index (BMI) of 25–40 kg/m , when used in conjunction with diet and exercise.
“More than half of the approximately 150 million adults in the U.S. with a BMI ranging from 25 kg/m2 to 40 kg/m2 are classified as overweight (BMI 25-30 kg/m2). Until now, many of them have not had access to prescription treatment options,” commented Harry L. Leider, MD, MBA, FACPE, Chief Medical Officer of Gelesis. “As the only prescription weight management product cleared for use by overweight adults with a BMI as low as 25 kg/m , we are eager to share new pivotal data which underscores the safety and efficacy of Plenity in patients in the overweight range as well as the broader range of obesity – up to a BMI of 35 kg/m . This new data highlights the unique opportunity Plenity creates to treat adults struggling to achieve a healthy weight earlier than previously possible with other prescription therapeutics.”
Details of the presentations are as follows:
Tuesday, November 5, 2019: 1:00 pm PT-3:00 pm PT, Drugs and Devices – New Interventions, and New Perspectives on Trusted Standbys
- Safety of Gelesis100 in Subjects Who Reached a Body Mass Index Below 27 kg/m2 in the GLOW Study; Louis J. Aronne, MD, FACP, Sanford I. Weill Professor of Metabolic Research, Weill-Cornell Medical College
- Safety of Gelesis100 in Overweight or Obesity: Comprehensive Analysis of the GLOW Study; Ken Fujioka, MD, Director of the Nutrition and Metabolic Research Center and the Center for Weight Management, Scripps Clinic
Thursday, November 7, 2019: 12:00 pm ET-1:30 pm PT, poster presentation
- Assessment of Early Intervention with Gelesis100 in Overweight and Mild Obesity in the GLOW Study; Frank L. Greenway, MD, Medical Director and Professor at the Pennington Biomedical Research Center, Louisiana State University System (T-P-3542)
About PlenityTM (Gelesis100)
Plenity is an oral, non-systemic, superabsorbent hydrogel which has received FDA clearance as an aid in weight management in adults with overweight and obesity with a BMI of 25–40 kg/m , when used in conjunction with diet and exercise. It is made by cross-linking two naturally derived building blocks, modified cellulose and citric acid, that create a three-dimensional matrix. Plenity particles rapidly absorb water in the stomach and homogenously mix with ingested foods. Rather than forming one large mass, it creates thousands of small individual gel pieces with the elasticity (firmness) of solid plant-based foods (e.g., vegetables) without caloric value. The Plenity hydrogel increases the volume and elasticity of the stomach and small intestine contents and induces a feeling of fullness and satiety. Once it arrives in the large intestine, the hydrogel is partially broken down by enzymes and loses its three-dimensional structure along with most of its absorption capacity. The released water is reabsorbed in the large intestine, and the remaining cellulosic material is eliminated through the body’s natural digestive processes. Plenity is considered a medical device because it achieves its primary intended purpose through mechanical modes of action consistent with mechanobiology constructs. For more information, visit myplenity.com.
Important Safety Information
- Plenity is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin or titanium oxide.
- Plenity may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully.
- Avoid use in patients with the following conditions: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); or complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility.
- Use with caution in patients with active GI conditions such as gastro-esophageal reflux disease (GERD), ulcers or heartburn.
- Overall, the most common treatment-related adverse events (TRAEs) were GI-related, with 38% of adults in the Plenity group and 28% of adults in the placebo group.
- The overall incidence of adverse events (AEs) in the Plenity group was no different from the placebo group.
Rx Only. For the safe and proper use of Plenity, refer to the Instructions for Use.
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of certain chronic diseases. In April 2019, Gelesis received FDA clearance for its lead product candidate, PlenityTM, as an aid for weight management in overweight and obese adults with a Body Mass Index (BMI) of 25-40 kg/m , when used in conjunction with diet and exercise. Gelesis anticipates Plenity will be broadly available by prescription in the U.S. in late 2020. Additionally, Gelesis is developing its second investigational candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel mechanotherapeutics based on the Gelesis platform technology are also being advanced in other GI inflammatory conditions, such as non-alcoholic steatohepatitis (NASH) and Chronic Idiopathic Constipation (CIC)).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials science, chronic disease research, and commercialization. Gelesis was co-founded by PureTech Health (LSE: PRTC), a biopharmaceutical company focused on the Brain-Immune-Gut (BIG) axis. For more information, visit gelesis.com or connect with us on Twitter @GelesisInc.
Contact:
Kathryn McNeil
+1 347 204 4226
pr@gelesis.com
Investigational candidate developed by Gelesis demonstrated significant 16 hour reduction in colonic
transit time in patients with chronic idiopathic constipation
First clinical study demonstrating super-absorbent hydrogel platform’s potential in this common
condition
BOSTON, May 19, 2019 — Gelesis, a biotechnology company at the forefront of developing
mechanobiology-based therapies to treat chronic diseases related to the gastrointestinal (GI)
system, announced the presentation of data from a clinical study demonstrating that GS500 prototype
(GS500/CSP01) provided a significant reduction in colonic transit time (CTT) in patients with chronic
idiopathic constipation (CIC) relative to placebo. The data were presented at Digestive Disease Week
2019, held in San Diego, California.
“One out of seven adults throughout the world suffer from chronic idiopathic constipation. This condition
can have a significant negative impact on quality of life,” said Dr. Braden Kuo, Gastrointestinal Unit in the
Massachusetts General Hospital (MGH) Department of Medicine. “The safety and efficacy results of this
study are intriguing and suggest further clinical evaluation in this very common, treatment resistant
condition would be both warranted and welcome.”
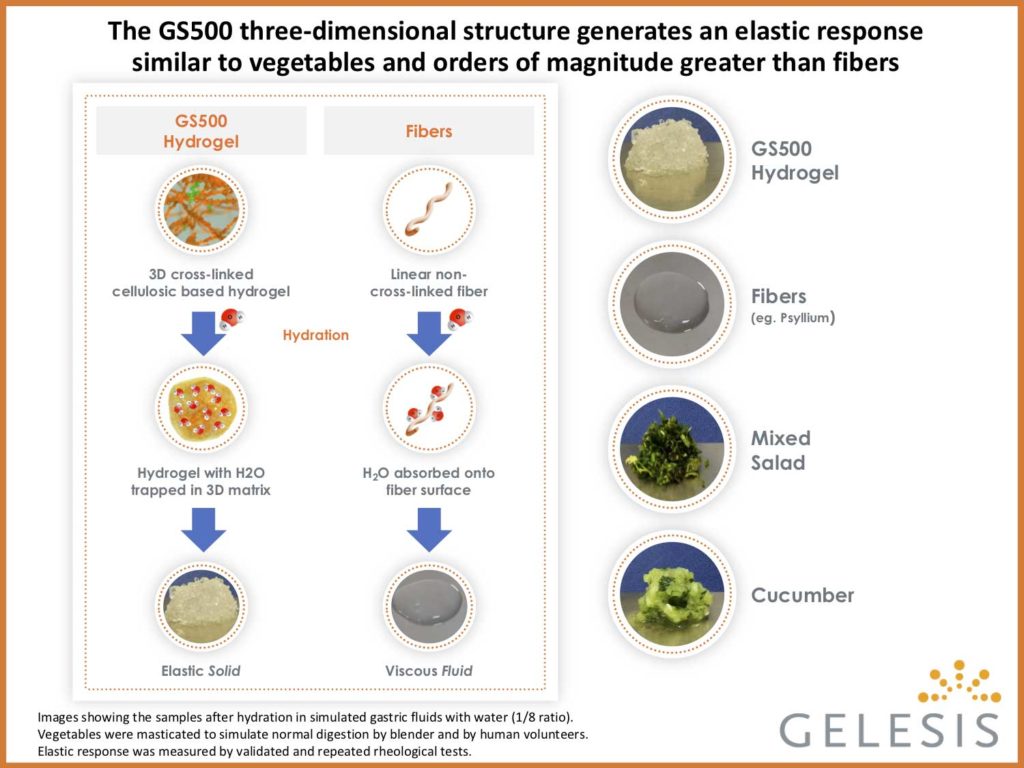
Gelesis’ proprietary hydrogel product candidates are orally administered and synthesized from two
naturally derived building blocks – modified cellulose cross-linked with citric acid – that create a three-
dimensional matrix designed to achieve specific mechanical properties (high elastic response) through the
gastrointestinal system. In order to assess the potential therapeutic benefits of the hydrogel’s specific
mechanical properties, modified cellulose, the main building block of GS500, was included as an active
control. This modified cellulose is a widely used soluble dietary fiber but lacks the three dimensional
structure of the superabsorbent hydrogel, and therefore creates significantly lower elastic response.
“The wireless motility capsule monitoring system allowed us to demonstrate that the superabsorbent
hydrogel, in contrast to modified cellulose alone or placebo, accelerated colonic transit time,” said Dr.
Kyle Staller, Center for Neurointestinal Health and Division of Gastroenterology at Harvard-affiliated
Massachusetts General Hospital. “This finding suggests that the three-dimensional structure of Gelesis’
hydrogel technology and specific elastic response may have contributed to the observed improvements
in colonic transit time over the active fiber control in this study.”
The primary end-point of this randomized, double-blind study was the change in CTT from pre-treatment
to post-treatment as measured by wireless motility capsules. The test involves swallowing a small data
recording device which transmits information to a wireless data receiver.
Two populations were evaluated separately, 27 subjects with CIC and 13 subjects with irritable bowel
syndrome with constipation (IBS-C). Patients were randomized into three treatment groups to receive 21
days of treatment with either GS500 (n=20), active control (modified cellulose, n=11) or placebo (n=9).
Each subject’s CTT was measured during the third week of treatment and compared to their baseline,
collected during 7 days of pre-treatment. Secondary outcome measures included improvement of
relevant gastro intestinal (GI) symptoms.
In the CIC population on treatment, colonic transit time was reduced by approximately 16 hours (~31%)
compared to baseline (P=0.02 compared to placebo). No statistically significant change was observed in
the placebo or the active control groups. No improvement was observed in the IBS-C population, as well
as no change in the reported GI symptoms which were the secondary endpoints. Two randomized patients
did not complete the study, one in the treatment group due to a GI related AE, and one in the placebo
group due to a faulty monitoring device. No serious adverse events were reported.
This pilot study of 40 subjects was powered to detect improvement in CTT (the primary end-point). Recent data suggest that colonic transit time influences gut health and a longer fecal retention time is associated with CIC symptoms and less microbiome diversity. Further studies are required to assess the effect of Gelesis’ hydrogel technology on symptom improvement.
About Chronic Idiopathic Constipation
Chronic idiopathic constipation (CIC) is a common gastrointestinal disorder. Its primary symptom is a low
frequency of bowel movements, which can cause significant discomfort and negative impact on quality-
of-life. CIC is estimated to affect between 15 and 25 percent of the general population in North America.
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic
diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the
GI pathway to potentially alter the course of chronic diseases. In April 2019, Gelesis received FDA
clearance for its lead product candidate, PLENITYTM. Gelesis is preparing to initiate a targeted U.S. launch
of PLENITY in the second half of 2019 and anticipates PLENITY will be broadly available by prescription in
the U.S. in 2020.
Additionally, Gelesis is developing its second candidate, Gelesis200, a hydrogel optimized for weight loss
and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel
mechanotherapeutics based on the Gelesis platform technology are also being advanced through a
pipeline (GS300, GS400, GS500) in other GI inflammatory conditions where gut barrier and gut
permeability potentially play a role, such as non-alcoholic steatohepatitis (NASH) and inflammatory bowel
disease (IBD). Recent preclinical data presented this year support the potential role of this novel hydrogel
platform technology in restoring gut barrier function and intestinal tissue health.
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials
science, chronic disease research and commercialization. Gelesis was co-founded by PureTech Health
(LSE: PRTC), a biopharmaceutical company focused on the Brain-Immune-Gut (BIG) Axis. For more
information, visit gelesis.com or connect with us on Twitter @GelesisInc.
Contact
Kathryn McNeil
+1 347 204 4226
kmcneil@gelesis.com
PureTech Health Conference Call on Gelesis FDA Clearance
PLENITY manufacturing facility will be the first factory to commercially produce medical super-absorbent
hydrogels synthesized from naturally derived building blocks
Targeted U.S. launch of PLENITY expected in 2H 2019, with broader U.S. availability by prescription in
2020
BOSTON, April 25, 2019 — Gelesis, a biotechnology company at the forefront of developing mechanobiology-based therapies to treat chronic diseases related to the gastrointestinal (GI) system, today announced it has received a non-dilutive $10.6 (€9.4) million grant to support the commercial manufacturing facility of PLENITY™, the company’s first commercial product, which was recently cleared by the FDA as an aid for weight management in adults with a Body Mass Index (BMI) of 25–40 kg/m2, when used in conjunction with diet and exercise.
“Gelesis is moving rapidly to build out its supporting infrastructure for the launch of PLENITY as a
prescription therapy, and this support from the Puglia Region and the European Community is a welcome
non-dilutive addition to our preparations,” said David Pass, chief operating officer and head of commercial
of Gelesis. “We are excited to build the first commercial manufacturing facility in the world capable of
producing super absorbent hydrogels synthesized from naturally derived building blocks, based on the
Gelesis core proprietary technology. This achievement is the result of many years of dedicated effort by
our multi-disciplinary engineering teams. We will continue to invest in our manufacturing processes and
capacity to meet demand for both commercial and clinical supply across our portfolio of hydrogel
therapies in development for chronic disease.”
Gelesis’ proprietary hydrogels are orally administered and synthesized from two naturally derived building
blocks – modified cellulose cross-linked with citric acid – that create a three-dimensional matrix to achieve
specific mechanical properties through the GI system.
The grant was provided by the Puglia Region, where Gelesis’ current material science research &
development and clinical supply manufacturing is located. The grant is intended to support the
development of a new commercial-scale manufacturing facility in the region. The grant uses funds
provided by the European Community via the Operative Program of the European Fund for Regional
Development (FESR), which supports small enterprises with research and industrialization-integrated
activity. This award marks the second grant awarded from the Puglia region of Italy. In 2011, Gelesis was
awarded a $1 million grant to scale up its laboratory and manufacturing facility near the town of Lecce in
Italy. Gelesis expects significant growth of its headcount as it continues its preparations for a targeted
U.S. launch in the second half of 2019, before broader U.S. availability by prescription in 2020.
About PLENITY™
PLENITY is an oral, non-systemic, superabsorbent hydrogel which has received FDA clearance as an aid in
weight management in overweight and obese adults with a BMI of 25–40 kg/m2, when used in conjunction
with diet and exercise. It is made by cross-linking two naturally derived building blocks, modified cellulose
and citric acid, that create a three-dimensional matrix. PLENITY particles rapidly absorb water in the
stomach and homogenously mix with ingested foods. Rather than forming one large mass, it creates
thousands of small individual gel pieces with the elasticity (firmness) of solid plant-based foods (e.g.,
vegetables) without caloric value. The PLENITY hydrogel mass increases the volume and elasticity of the
stomach and small intestine contents and induces a feeling of fullness and satiety. Once it arrives in the
large intestine, the hydrogel is partially broken down by enzymes and loses its three-dimensional structure
along with most of its absorption capacity. The released water is reabsorbed in the large intestine, and
the remaining cellulosic material is expelled in the feces. PLENITY is considered a medical device because
it achieves its primary intended purpose through mechanical modes of action consistent with
mechanobiology constructs. For more information, visit myplenity.com.
Important Safety Information
-
PLENITY is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid,
sodium stearyl fumarate, gelatin or titanium oxide
-
PLENITY may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for
Use carefully
-
Avoid use in patients with the following conditions: esophageal anatomic anomalies, including
webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); or
complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility.
-
Use with caution in patients with: active GI conditions such as gastro-esophageal reflux disease
(GERD), ulcers or heartburn.
-
Overall, the most common treatment related adverse events (TRAEs) were GI-related with 38%
of adults in the PLENITY group and 28% of adults in the placebo group.
-
The overall incidence of AEs in the PLENITY group was no different than the placebo group
Rx Only. For the safe and proper use of PLENITY, refer to the Instructions for Use.
About Gelesis
Gelesis is developing a novel hydrogel platform technology to treat overweight and obesity and chronic diseases related to the GI pathway. Gelesis’ proprietary approach is designed to act mechanically in the GI pathway to potentially alter the course of chronic diseases. In April 2019, Gelesis received FDA clearance for its lead product candidate, PLENITY™, as an aid for weight management in overweight and obese adults with a Body Mass Index (BMI) of 25-40 kg/m2, when used in conjunction with diet and exercise. Gelesis is preparing to initiate a targeted U.S. launch of PLENITY in the second half of 2019 and anticipates PLENITY will be broadly available by prescription in the U.S. in 2020. Additionally, Gelesis is developing its second investigational candidate, Gelesis200, a hydrogel optimized for weight loss and glycemic control in patients with type 2 diabetes and prediabetes. Novel hydrogel mechanotherapeutics based on the Gelesis platform technology are also being advanced through a pipeline in other GI inflammatory conditions where gut barrier and gut permeability potentially play a role, such as non- alcoholic steatohepatitis (NASH) and inflammatory bowel disease (IBD).
The Gelesis executive and advisory team includes some of the world’s leading experts in obesity, materials
science, chronic disease research and commercialization. Gelesis was co-founded by PureTech Health
(LSE: PRTC), a biopharmaceutical company focused on the Brain-Immune-Gut (BIG) axis. For more
information, visit gelesis.com or connect with us on Twitter @GelesisInc.
Contact:
Kathryn McNeil
+1 347 204 4226
kmcneil@gelesis.com
Tom Donovan
+1 857 559 3397
tom@tenbridgecommunications.com