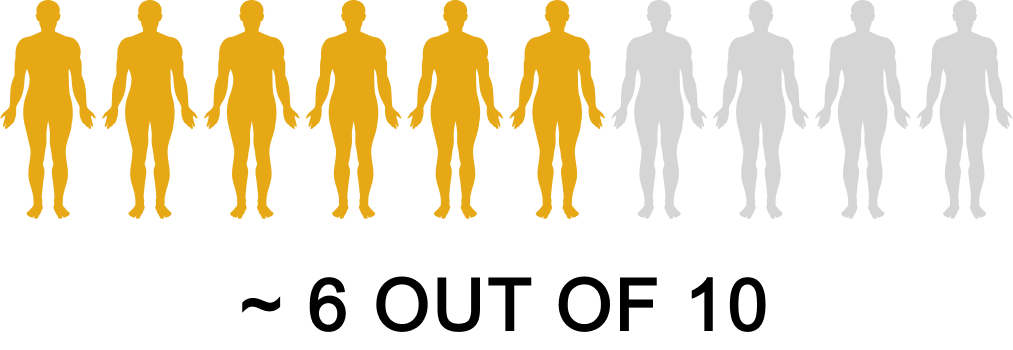X
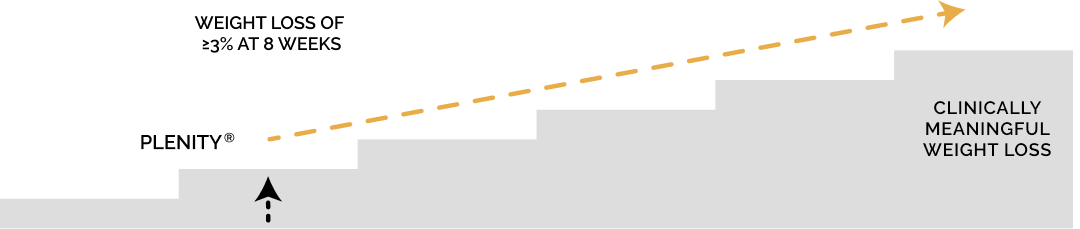
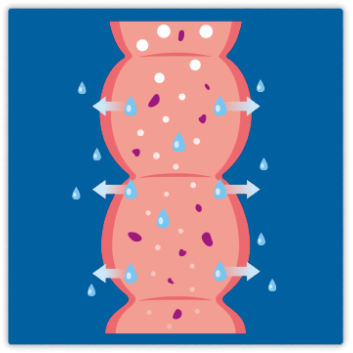
Plenity is an aid for weight management in adults who are overweight or obese and have a body mass index (BMI) of 25 to 40 kg/m , when combined with diet and exercise.
IMPORTANT SAFETY INFORMATION
- Patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium dioxide should not take Plenity.
- To avoid impact on the absorption of medications:
- For all medications that should be taken with food, take them after starting a meal.
- For all medications that should be taken without food (on an empty stomach), continue taking on an empty stomach or as recommended by your physician.
- The overall incidence of side effects with Plenity was no
different than placebo. The most common side effects were diarrhea, distended abdomen, infrequent bowel movements, and flatulence. - Contact a doctor right away if problems occur. If you have a severe allergic reaction, severe stomach pain, or severe diarrhea, stop using Plenity until you can speak to your doctor.
Rx Only. For the safe and proper use of Plenity or more information, talk to a healthcare professional, read the Patient Instructions for Use, or call 1-844-PLENITY.